This article comprehensively explores the recent advancements in bacteriophage-based biosensors for rapidly detecting cancers. It offers a detailed overview of these cancers, emphasizing the critical need for early detection and the role of innovative biosensor technologies. Furthermore, the article delves into clinical applications and provides a comparative analysis with traditional detection methods. It addresses current challenges and envisages future research directions in biosensor technology. This work highlights the potential of bacteriophage-based biosensors as a significant advancement in cancer diagnostics.
Author ID: 2024024866
1. Introduction Bacteriophage Endolysin-Based Biosensors
1.1. Bacteriophage
The use of bacteriophage biosensors in cancer detection represents notable progress in the accuracy and effectiveness of diagnosis. These biosensors use the specific nature of bacteriophages, which are viruses that infect bacteria and are cleverly modified to target cancer cells [1]. This innovation comes from the remarkable futures of bacteriophages, including the ability to detect specific markers on cancer cells with high affinity and selectivity, therefore recognizing different types of cancer [2]. This method dramatically improves the accuracy of detecting cancer, particularly in its early stages when it’s crucial to detect cancerous cells quickly for effective treatment and better patient outcomes.
Bringing bacteriophage biosensors into the cancer diagnosis process is a significant change from traditional methods, providing a quicker and more sensitive option. The technology in these biosensors is based on sophisticated genetic engineering, which allows them to identify a wide range of cancer markers with great precision. This precision reduces the possibility of false positives, a common issue with conventional cancer screening. As ongoing research and development progress, bacteriophage biosensors become a crucial part of oncology, potentially transforming how cancer is diagnosed and managed.
1.2. Endolysins protein
The science behind bacteriophage endolysins lies at the heart of their innovative application in cancer detection. Bacteriophage endolysins, which are enzymes to break down target cell walls, applied in cancer detection due to their remarkable abilities (Fig. 1) [3]. They are engineered to identify specific cancer cells, facilitating the release of markers critical for early cancer recognition and treatment.
Nowadays, developing endolysin-based biosensors that are highly sensitive and capable of rapid cancer cell identification leads to advancement in the phage sensor field. Integrating endolysins with biosensor technology creates a powerful tool for oncology diagnostics, offering a non-invasive and efficient alternative to traditional methods. The precision of these biosensors in identifying cancer markers ensures a high degree of accuracy, reducing the likelihood of false positives. As research continues to enhance the stability and efficacy of endolysin-based biosensors, their potential in transforming cancer diagnostics grows, promising a future where early detection and effective treatment of cancer are more attainable.
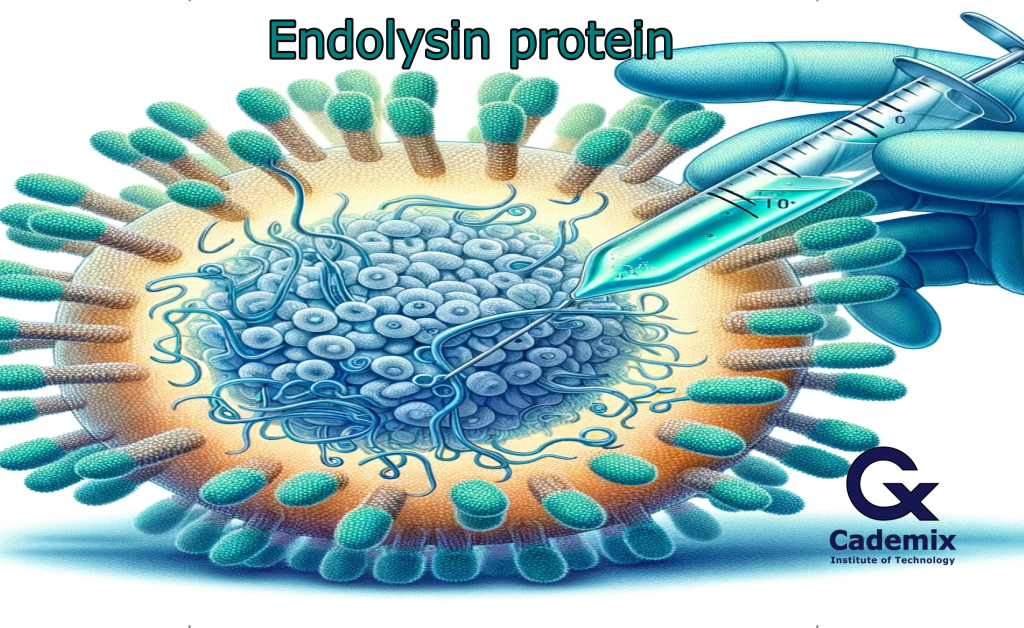
Fig.1. The schematic of Endolysin protein
2. The Critical Requirement for Early Cancer Detection
2.1. Overview of Cancer Progression and Diagnosis Challenges
The progression of cancer, a complex and multifaceted process, presents significant challenges in diagnosis and treatment. Cancer development typically follows a series of stages, beginning with the initial genetic mutation in cells, leading to uncontrolled cell growth and potentially metastasis, where cancer spreads to other parts of the body. Early detection is crucial as it significantly improves the chances of successful treatment. However, one of the primary challenges in cancer diagnostics is the identification of malignancies at an early stage when symptoms are often non-specific or absent. Traditional diagnostic methods, while effective in later stages, can miss early, subtle signs of cancer, highlighting the need for more sensitive and precise diagnostic tools.
The diagnosis of cancer is further complicated by its heterogeneity. Different types of cancers exhibit varied behaviors and responses to treatment, necessitating personalized diagnostic approaches. This requirement for specificity often makes it difficult to develop universal screening methods that are effective across all cancer types. Additionally, patients may experience dangers and discomfort from the intrusive nature of some diagnostic procedures, such as biopsies, emphasizing the need for reliable but non-invasive diagnostic methods.
Developing new diagnostic tools that can identify cancer early on is necessary to address these issues. Integrating advanced biosensors and molecular diagnostic tools promises a new era in cancer detection, offering the sensitivity and specificity needed to identify cancer in its early stages. These technologies hold the potential to transform cancer diagnostics, enabling timely and personalized treatment and ultimately improving patient outcomes. As research advances in this field, the goal of early, accurate, and non-invasive cancer diagnostics becomes more available.
2.2. Role of Biosensors in Transforming Cancer Diagnostics
The role of biosensors in cancer diagnostics is crucial, marking a significant advancement in the early detection and management of cancer [3]. These sophisticated devices are designed to detect specific biological markers associated with cancerous cells, offering a level of sensitivity and specificity that traditional diagnostic methods often lack. The ability of biosensors to identify minute quantities of cancer markers in biological samples, such as blood or tissue, facilitates the early detection of cancer, a critical factor in improving treatment outcomes. This early detection is particularly valuable in cancers that do not exhibit clear symptoms until advanced stages, allowing for interventions at an stage when the disease is more manageable and treatment more likely to be successful.
Biosensors also bring the advantage of non-invasive testing, which is less traumatic for patients compared to traditional methods like biopsies. This non-invasive nature not only enhances patient comfort but also encourages more frequent monitoring, which is crucial for tracking the progression of the disease or the effectiveness of treatment. Integrating advanced materials and technologies in biosensors, such as nanotechnology and microfluidics, further improves their accuracy and reduces the time needed for analysis, enabling faster clinical decision-making.
Moreover, the adaptability of biosensors to various cancer types makes them a versatile tool in oncology. Biosensors designed to detect specific types of cancer can be used in personalized medicine approaches, providing individualized diagnostic and monitoring solutions. As research and development in biosensor technology continue to advance, these devices are expected to become integral in cancer diagnostics, revolutionizing the way cancers are detected, monitored, treated and ultimately leading to improved patient survival rates and quality of life.
3. Clinical Applications of Bacteriophage Biosensors
3.1. Real-world Implementation in Cancer Diagnostics
The application of cutting-edge biosensors in cancer diagnostics represents an evolution in how cancer is identified and treated rapidly. Clinical trials and research studies demonstrate the efficacy of these biosensors, showcasing their potential to detect various cancer types with high precision and reliability. Such implementation in clinical settings is not just a theoretical advancement but a practical solution addressing the urgent need for early cancer detection. These biosensors, designed to identify specific biomarkers associated with cancerous cells, enable clinicians to diagnose cancer at earlier stages than traditional methods allow. This early detection is crucial in increasing the effectiveness of treatment and improving patient survival rates.
Moreover, incorporating biosensor technology into existing diagnostic frameworks reshapes the landscape of cancer care. Hospitals and diagnostic centers are gradually adopting these technologies, appreciating their accuracy, speed, and patient comfort benefits. The non-invasive nature of many biosensor-based tests, such as blood-based assays, makes them more patient-friendly and less risky than invasive procedures like biopsies. This aspect is particularly important in encouraging regular monitoring and follow-up, which is essential for patients with a high risk of cancer or those undergoing treatment.
As these biosensors are refined and validated for clinical use, their role in cancer diagnostics is set to expand further. Their flexibility for unique disease types makes them suitable for personalized medicine, aligning with the current trend toward more individualized cancer treatment approaches.
This real-world application of biosensor technology in cancer diagnostics represents a significant leap in medical science and offers hope for a future where cancer can be detected and treated more effectively, ultimately saving more lives.
3.2. Patient-Centric Advantages
The application of biosensor technology in cancer diagnostics creates a revolution and a new era of patient-centric advantages, significantly enhancing the patient experience in medical care. One of the primary benefits of these advanced biosensors is their non-invasive nature. Unlike traditional diagnostic methods such as biopsies, which can be painful and carry risks of complications, biosensors often require only simple, non-intrusive samples like blood or urine. This non-invasiveness not only reduces patient discomfort and anxiety but also lowers the risk of infection and other procedural complications. Patients are, therefore, more likely to follow screening and monitoring plans on a regular basis, which is essential for early cancer detection.
Furthermore, biosensor technology offers the advantage of rapid and accurate results, reducing the often stressful waiting period associated with cancer diagnosis. The ability of these devices to provide swift feedback is vital in fast-tracking the initiation of treatment, a critical factor in improving cancer prognosis. This timeliness also allows for more efficient and dynamic management of the disease, with the potential to adjust treatment plans in response to real-time changes in the patient’s condition.
In addition to these benefits, integrating biosensors into cancer care aligns with the growing trend toward personalized medicine. By modifying diagnostics and treatment to the individual’s specific cancer type and genetic makeup, biosensors contribute to more targeted and effective therapy regimens. This personalized approach enhances treatment efficacy and minimizes unnecessary side effects, improving the overall quality of life for patients undergoing cancer treatment. As biosensor technology continues to evolve, its role in fostering patient-centered care in oncology is poised to grow, offering a more compassionate, efficient, and effective approach to cancer diagnosis and management.
In a separate study, Han et al. developed an innovative phage-based sensor designed to detect cancer at its early stages. This advancement represents a significant leap in cancer diagnosis, emphasizing the potential of phage technology in medical applications. The early detection of cancer is crucial for effective treatment, and the introduction of such a sensor could greatly improve patient outcomes. However, while this novel approach holds promise, it may also face challenges in clinical implementation, such as ensuring accuracy and specificity in diverse patient populations, and navigating the regulatory landscape for new medical technologies. This balance of pioneering potential with practical considerations reflects the ongoing evolution and complexity of cancer research and diagnostics.
4. Detecting Different Analytes
Biosensors based on bacteriophages are not limited to the detection of cancer. They are also capable of recognizing other proteins and peptides, such as bacteria.
At the Interdisciplinary Research Institute of Grenoble (IRIG), scientists designed a cutting-edge method in the field of bacteriophage binding to gold nanoparticles for more bacterial capture. The study investigates how combining purification techniques with both covalent and physisorption methods enhances the attachment of bacteriophages to gold surfaces. By utilizing these approaches together, the research aims to maximize bacteriophage binding efficiency to gold, pushing the boundaries of geometric limits. This innovative approach has the potential to significantly improve various applications, such as biosensing and nanomedicine, by increasing the precision and effectiveness of bacteriophage interactions with gold materials [4].
SyntBiolab, a leading institution in biotechnology, has pioneered the use of bacteriophages to detect foodborne pathogens, notably Escherichia coli (E. coli), a common and potentially harmful bacterium found in food. This innovative approach leverages the natural targeting capability of bacteriophages, which are viruses that specifically infect bacteria, identify and quantify E. coli presence in food samples. By employing bacteriophages that have a high affinity for E. coli, SyntBiolab’s method offers a rapid, accurate, and non-invasive way to ensure food safety. This technology not only enhances the efficiency of detecting contamination in the food supply chain but also significantly reduces the risk of foodborne illnesses, demonstrating SyntBiolab’s commitment to employing cutting-edge science for public health advancements [5].
Z.Yousefniayejahromi et al. developed the novel Zeno phage sensor to identify Staphylococcus aureus in synovial fluid with a linear range from 101 to 104 CFU/ml with a detection limit of 6 CFU/mL [6].
Han et al. developed a label-free cytosensor technology that offers numerous advantages. This innovative approach employs specific peptides derived from a phage library to create a highly specific and sensitive electrochemical impedance cytosensor. Some key advantages include label-free detection, which eliminates the need for costly chemical labeling, high specificity towards target cells or molecules, sensitive detection of low concentrations, and versatile applications in biotechnology and diagnostics. However, there are challenges, such as the complexity of phage library selection, the importance of accurate sample preparation, initial setup and maintenance costs, and potential limitations in the target range [7].
5. Challenges and Limitations in Current Biosensor Technologies
The field of biosensor technology, despite its remarkable progress in cancer diagnostics, faces several challenges and limitations that need addressing for its full potential to be realized. One of the primary challenges lies in the accuracy and sensitivity of these devices. While biosensors shown promising results in detecting cancer markers, ensuring consistent accuracy across different types of cancers and stages of disease remains a hurdle. Variability in biomarker expression among patients can lead to false negatives, potentially delaying diagnosis and treatment. Moreover, in early-stage cancer diagnosis, biosensor sensitivity must be precisely calibrated to detect low amounts of cancer markers when biomarker levels may be low.
Another significant limitation is the reproducibility of biosensor technologies. Manufacturing biosensors that maintain consistent performance across large batches is a challenge, essential for their widespread clinical use. Ensuring that each biosensor device works with the same level of efficiency and accuracy is vital for their reliability and trustworthiness in clinical settings. Additionally, integrating these advanced technologies into existing healthcare systems poses logistical and infrastructural challenges. Adapting current diagnostic pathways to incorporate biosensor technology requires substantial investment, training, and systemic changes, which can be a barrier to widespread adoption.
Furthermore, the long-term stability and shelf-life of biosensor devices are areas of concern. The components of biosensors, especially biological elements, may degrade over time, affecting the device’s performance. Ensuring these devices remain stable and functional over their intended shelf-life is crucial for their practical use in clinical settings.
While biosensor technologies hold immense promise in revolutionizing cancer diagnostics, overcoming these challenges is key to their successful implementation. Addressing issues related to accuracy, reproducibility, scalability, stability, and regulatory compliance will be crucial in harnessing the full potential of biosensors in clinical practice, ultimately leading to improved patient care and outcomes in cancer treatment.
6. Future Perspectives
6.1. Innovations on the Horizon
Significant innovations in biosensor technology are on the horizon, promising to revolutionize cancer diagnostics. The advent of nanoscale biosensors is a crucial development, enhancing the sensitivity and specificity of cancer detection. These nano biosensors, utilizing nanomaterials’ expansive surface area and unique properties, can detect cancer markers at exceptionally low concentrations, crucial for early-stage diagnosis. This advancement not only promises greater accuracy in identifying malignancies but also paves the way for detecting a broader range of cancer types at their nascent stages.
Another groundbreaking innovation is integrating artificial intelligence (AI) with biosensor technology. AI’s capability to analyze and interpret complex data patterns will significantly improve the diagnostic accuracy of biosensors. This fusion of AI and biosensors is expected to lead to smarter, more efficient diagnostic tools capable of providing real-time, actionable insights for clinicians. The potential for multiplexing, enabling simultaneous detection of multiple cancer markers, further underscores the transformative impact of these emerging technologies. As these innovations progress, they promise to usher in a new era in cancer diagnostics, marked by enhanced precision, efficiency, and a shift towards more personalized and proactive cancer care.
6.2. Path Forward for Bacteriophage-Based Biosensors
Bacteriophage-based biosensors for cancer diagnosis have a bright future ahead of them thanks to ongoing innovation and teamwork in research. The future development of these biosensors hinges on enhancing their specificity and sensitivity, ensuring that they can reliably detect a wide range of cancer types at early stages. This requires ongoing research into optimizing bacteriophage engineering, focusing on refining their ability to target and bind to specific cancer markers. Collaborative efforts between biotechnologists, oncologists, and engineers are essential in this endeavor, as they bring together diverse expertise to address the multifaceted challenges of cancer diagnostics.
Moreover, integrating these biosensors into clinical practice is a critical step in realizing their potential. This involves rigorous clinical trials to validate their efficacy and safety, followed by developing guidelines for their use in medical settings. A focus on these biosensors’ cost and scalability is also necessary to guarantee that various healthcare systems and patient populations can use them. Bacteriophage-based biosensors have the potential to be a key component in the early diagnosis and treatment of cancer, providing a novel approach in the ongoing battle against this widespread illness if certain obstacles are resolved.