Chemical regulations allow companies and users to handle chemicals more safely. Apart from that, there are some other benefits that these chemical regulations may offer to society and industry. REACH, CLP, BPR, POPs, PIC, CAD, and CMD are some important European Union chemical regulations. This article will highlight the important European Union chemical regulations, and what job seekers should know about it. The main focus is REACH and CLP regulations. The four steps of the REACH regulation: registration, evaluation, authorization, and restriction will be covered in detail. Similarly, this article will assist you in getting a general idea of European Union regulations on classification, labelling, and packaging.
Rosemary Salin, Cademix Institute of Technology
Introduction
Chemicals have become a vital part of our daily lives. But, there are some hazardous chemicals. They can be harmful to our health and the environment. As a result, chemical regulations are necessary to assure the safety of the worker and the environment. There are regional regulations, as well as some international actions for the regulation of chemicals.
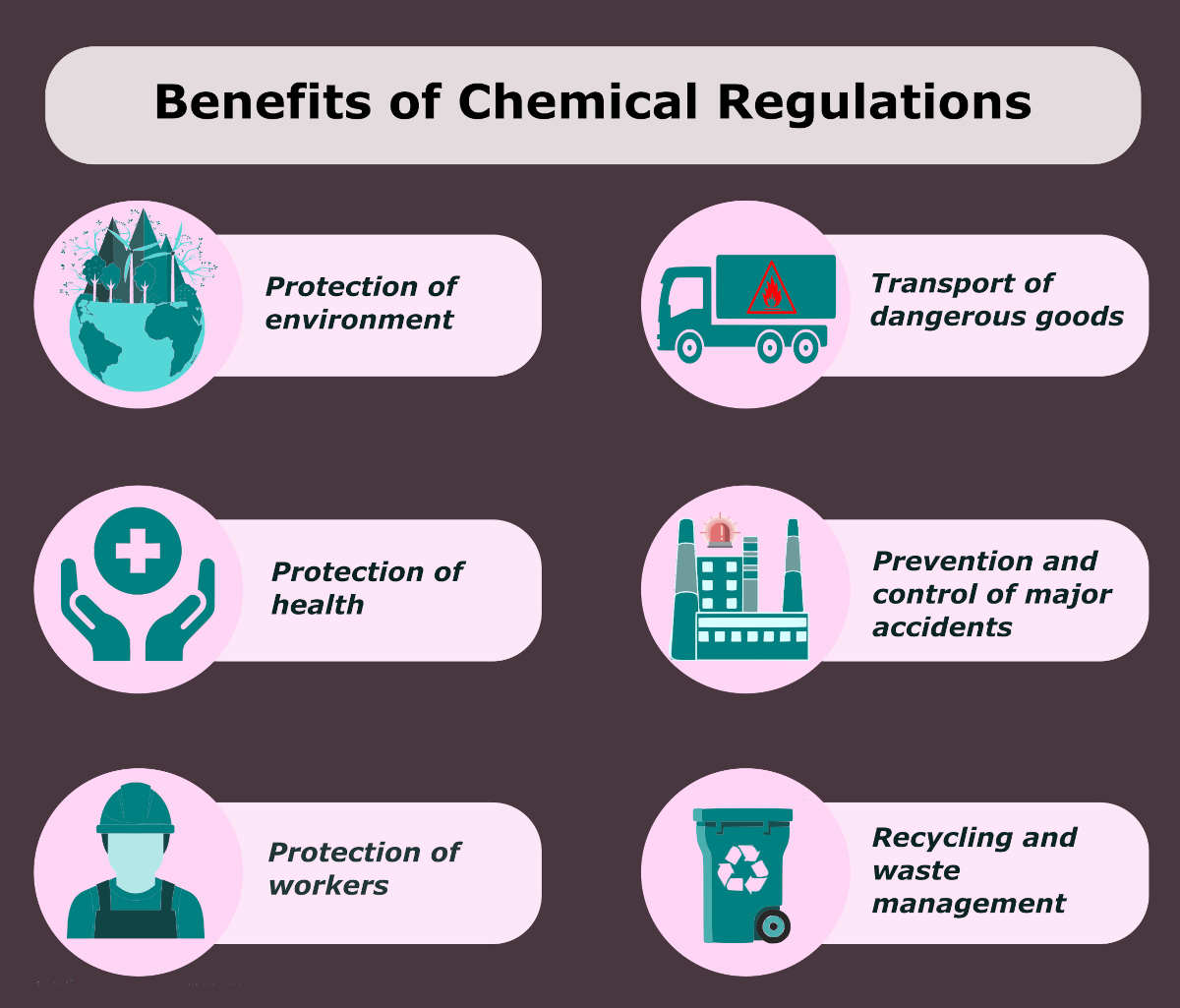
Chemical regulations ensures environmental protection, control of emissions from industry, worker safety, and safety of the user. On top of that, it helps in avoiding major accidents, safe transport of harmful products, and waste management. For instance, information on risk control in the workplace can help to prevent emissions. In addition, it allows safe transit and storage of chemicals. Similarly, details about the chemical content of all products helps in recycling and safe waste disposal.
European Union Chemical Regulations

Europe has wide range of chemicals regulation, led by REACH and CLP. They ensure a high level of safety for human health and the environment. However, specific chemical groups, such as biocides, pesticides, pharmaceuticals, and cosmetics, have their own regulation. On top of that, there are some other regulations and directives like PIC, POPs, CAD, CMD, and so on. In this article, we will discuss the REACH and CLP regulations in detail. These two regulations are the two highly important European Union chemical regulations. So, the job seekers should a basic knowledge about them. Moreover, you will see some other regulations like PIC regulation, POPs regulation, and BPR. As well as, we will also discuss some directives like CAD and CMD. Along with that, there will be examples of some important regulatory IT tools that are used in EU chemical sector.
Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH)
REACH is one of the most important European Union chemical regulations. It enters into force on June 1, 2007. The regulation also created the European Chemicals Agency (ECHA). ECHA is in charge of REACH’s technical, scientific, and legislative components. REACH controls all chemicals with some exceptions. It applies to all chemicals imported or produced in the EU. The major goals of REACH are to:
- Provide protection for human health from the use of chemicals.
- Provide protection for the environment from the use of chemicals.
- Allow free transit of chemicals on the EU market.
- Improve EU chemicals industry’s innovation and competitiveness.
- Reduce the number of hazard assessment tests on animals. It is done by promoting other methods for the hazard assessment.
The necessity to share all the details about a chemical up and down the supply chain is a major aspect of the REACH regulation. This make sure that makers, importers, and users are all aware the safety of the items they are handling. Companies must identify and manage the risks arising or likely to arise from the chemicals they produce. Moreover, they must show ECHA how these chemicals can be used safely. In addition, they must convey the risk control methods to the users. REACH regulation involves four steps: Registration, Evaluation, Authorisation, and Restriction.
Registration
Companies must identify the properties and uses of the chemicals they synthesise or import. Prior registration is necessary if a company wants to produce or import a substance one tonne or more per year. They must also analyse the possible risks posed by the chemical. Then, this data must be sent to ECHA through a registration dossier. It must include the details like hazard information and safe handling of the substance. Similarly, they must use a safety data sheet to inform the risk control measures to the user. Pharmaceuticals or radioactive chemicals that are currently controlled by other laws are excluded from REACH in part or whole. Registration is based on the “one substance, one registration” principle. This means that manufacturers and importers of the same material must register jointly.
Evaluation
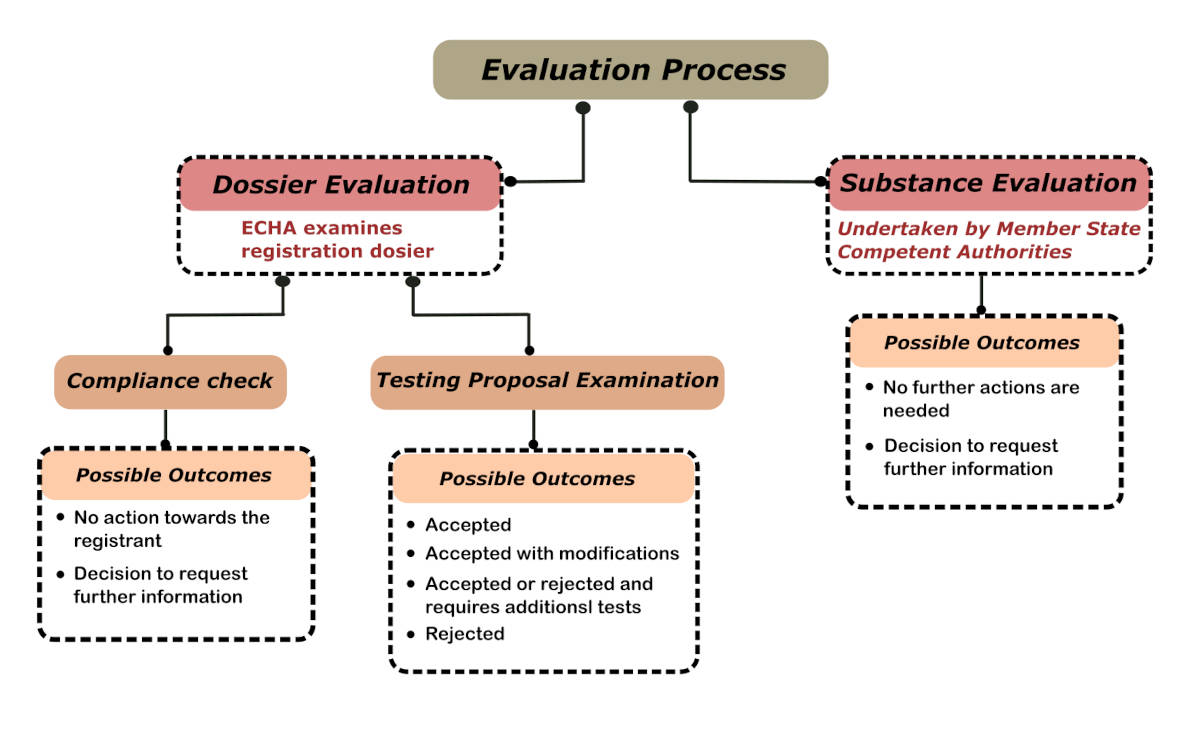
After registrations are received, ECHA will evaluate them. During the evaluation process, ECHA will verify whether registrations meet the criteria of REACH regulation. ECHA and the Member States analyse the data given by companies. Thus, they will assess the quality of the registration dossiers and testing plans. Finally, they decide whether a chemical is safe or not.
The two types of evaluation are Dossier evaluation and substance evaluation. The dossier evaluation confirms that no needless animal testing or costs are made. It also checks the compliance of the registration dossier with the registration requirements. Substance evaluation is done when there is reason to suspect that a chemical poses a risk to human health or the environment. In order to check the suspected problems, ECHA will ask the registrants of the product to submit more details.
Authorisation
Authorisation is a licensing system. The goal of the authorisation process is to replace substances of very high concern (SVHCs) with less harmful compounds. The identification of a chemical as SVHC is the first stage in the authorisation process. Following that, this SVHC is added to the candidate List of ECHA. ECHA evaluates the compounds on the candidate List regularly. Followingly, ECHA suggests the chemicals that should be added to the Authorisation List. Anyone who wants to keep using a substance on the authorisation list must apply for approval from ECHA. Then, ECHA will make the final decision on whether to give the authorisation or not.
Restriction
Restrictions are in place to protect human health and the environment from the severe risks caused by chemicals. Manufacturing, sales, and the usage of harmful compounds and mixes can all be restricted. Moreover, the limits might vary from a complete ban to specific sales restrictions. On the request of the European Commission, Member States or ECHA can start the restriction process.
Classification, Labelling, and Packaging (CLP) in European Union chemical regulations
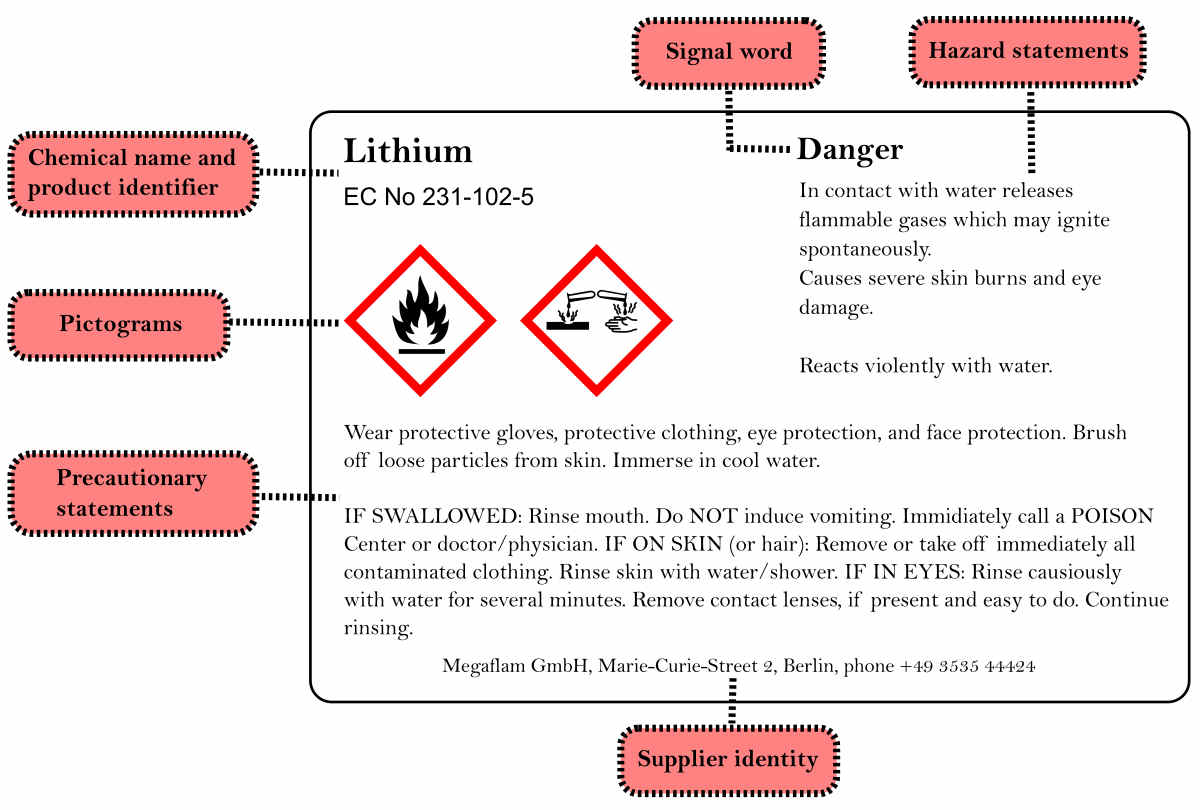
CLP Regulation is another major European Union chemical regulation. It is based on the Globally Harmonized System of the United Nations (GHS). It established new classification criteria, European hazard symbols (pictograms), and risk and safety statements. Before putting products on the market, companies must properly classify, label, and package them, according to the legislation. It tries to protect workers, customers, and the environment by labelling the possible risks.
Classification
Classification serves as the basis for hazard communication. Physical, health, environmental, and additional hazards are the four hazard classes under CLP. Firstly, the company should evaluate the properties of the substance. They are then classified into various hazard classes. This self-classification is done by the chemical manufacturer, importer, or downstream user.
Harmonised classification and labelling
Harmonized classification and labelling(CLH) ensures sufficient risk control methods for the hazards of great concern (carcinogenicity, mutagenicity, reproductive toxicity (CMR), and respiratory sensitizers). As a result, manufacturers, importers, and downstream users of such compounds must apply the CLH.
Labelling
The identified hazards must be conveyed after a substance has been classified. Hazard labelling allows to identify hard classes via the use of labels and safety data sheets. As a result, it helps in alerting the presence of a hazard to the user. Similarly, it reminds the necessity to manage the related risks. For each hazard class, CLP gives precise standards for labelling components such as pictograms, signal words. Moreover, CLP provides standard statements for hazard, prevention, response, storage, and disposal.
Packaging
CLP sets basic packaging standards to allow the safe transport of harmful chemicals and mixtures. The packing materials must be strong. Because, a strong packing material will help in safe transport of chemicals. Similarly, to ensure safe storage of chemicals, packing material should be durable. Above all, it should be resistant to harm caused by the contents. Furthermore, the packaging of a product sold to the public must neither attract kids nor should it mislead users. Moreover, packaging must not have a similar a design used for foodstuff or animal feedstuff, or medicinal or cosmetic products.
So far, we have seen the two major European Union chemical regulations. Now we will discuss some other European chemical regulations such as BPR, PIC regulation, POPs regulation, CAD, and CMD. Furthermore, you will see some examples of regulatory IT tools used in the European chemical sector.
Biocidal Products Regulation (BPR)
The Biocidal Products Regulation (BPR) is another European Union regulation. It governs the marketing and use of biocidal products. This regulation intends to enhance the operation of the EU’s biocidal products market. At the same time, it ensures safety of users, workers, and the environment. The following are the some definitions related to biocidal products.
- Active substance: a substance that has activity on or against harmful organisms.
- Biocidal products: any chemical that contains or produces one or more active compounds.
- Treated articles: articles that have been treated with or include one or more biocidal products.
All biocidal products must get approval from the ECHA before being placed on the EU market. This is the main criterion of BPR legislation. However, the active ingredients in that biocidal product must be already approved. We can market and use biocidal products with active substances under review. However, it is allowed only till the formation of approval decision on the active substance. Similarly, temporary approval is given for marketing products with new active substances that are currently being assessed.
Persistent Organic Pollutants (POPs) Regulation
POPs are chemicals that:
- remain intact for long periods (many years);
- spread all over the environment as a result of natural processes involving soil, water, and, most notably, air (mobility; long-range transport);
- deposit in the fatty tissue of living organisms, including humans;
- are found at higher concentrations at higher levels in the food chain; and
- are toxic to both humans and wildlife.
The Stockholm Convention and the Aarhus Protocol govern POPs globally. The POPs Regulation uses these pieces of law in the European Union. It aims to protect human health and the environment by applying particular control measures such as:
- ban or highly restrict POP production;
- prohibit or control marketing of POPs;
- restrict the use of POPs;
- reduce the environmental emission of POPs produced as industrial byproducts;
- ensure the safe storage of restricted POP stocks; and
- ensure the eco-friendly disposal of POP-containing waste.

Prior Informed Consent (PIC) in European Union chemical regulations
The PIC regulation controls the sale of certain hazardous substances that are either prohibited or highly controlled in the EU. It imposes obligations on EU companies who want to export or import these chemicals. Besides, it promotes shared responsibility and cooperation in the worldwide trade of hazardous chemicals. In addition, it gives directions to importing nations on how to securely store, transport, use, and dispose of hazardous goods. Thus, it helps to protect human health and the environment.
Companies seeking to export chemicals stated under PIC to non-EU nations must report their intention to national authorities. Each year, importers and exporters of PIC chemicals must report the exact quantities of the chemical shipped to or from each non-EU country during the previous year. And, before the export may take place, make a confirmation on export permission.
Chemical Agents Directive (CAD) and Carcinogens and Mutagens Directive (CMD)
The two laws, CAD and CMD, are major elements of the EU’s system for protecting workers. These two directives uses occupational exposure limit (OEL) values. OELs are regulation values that reflect levels of exposure that are assumed to be safe (health-based) for a chemical substance in the air of a workplace. Moreover, setting limitations on a certain substance’s exposure helps companies in protecting the health of workers. Taking action against harmful products is a top concern for safety of workers in the European Union.
Chemical Agents Directive (CAD)
CAD sets the minimum criteria for protecting workers from hazards arising from the use of chemical agents at work. It sets OELs and biological limit values. A chemical agent is by definition any chemical compound that exists in its natural state or produced, used, or emitted (including waste) by any work activity. As a result, the directive also concerns the assessment of emissions and process wastes.
Carcinogens and Mutagens Directive (CMD)
CMD sets the basic criteria for protecting workers from hazards from the workplace by exposure to carcinogens and mutagens. It specifies precautionary and protective measures, as well as exposure limits. A carcinogen is a substance that causes or has the potential to cause cancer. Whereas, a mutagen is a substance that may cause harm to genetic material in cells. It may leads to heritable genetic damage or cancer.
So far, we have discussed several key EU chemical regulations. Every chemical sector job seeker should be aware of these regulations. Let us now look at some IT tools related to these regulations.
Software and IT Tools related to European Union Chemical Regulations
There are some software and tools that helps to collect, store, submit, process, and manage data. We discuss some of the important tools in the following.
REACH-IT
REACH-IT is the primary IT system that enables industry, Member State competent authorities, and the European Chemicals Agency to properly submit, process, and manage data and dossiers. These three parties have access to certain feature of REACH-IT. They can utilise these functions to meet REACH and CLP requirements. It also provides a secure data transfer channel between these three parties. Thus, it helps in the coordination of data processing and dossier analysis.
IUCLID
IUCLID is a software tool that collects, stores, maintains, and exchanges data on chemical compounds’ basic and hazardous properties. This is a free software. It is highly beneficial to chemical sector corporations and government agencies. Hence, it is a critical tool for the chemical industry in meeting REACH data submission requirements.
R4BP 3
We make all biocides applications through R4BP 3. It is a dedicated IT platform for biocides. It offers functions that allow industry and authorities to comply with legislations. Moreover, it also helps in exchanging information. First step for application is to create a IUCLID dossier. Then, you can submit it to ECHA and the national authorities through R4BP 3.
SPC Editor
ECHA has developed an online tool called SPC (summary of the product characteristics) Editor to help companies in submitting their applications for authorization of biocidal goods, either on a national or European level. The tool is completely compatible with R4BP 3. Applicants can use this tool to generate SPC for single items, product families, and family members.
SAP EHS (Environment, Health, and Safety Management)
SAP EHS is a software that assists companies in detecting and mitigating organisational hazards. It helps in the management of industrial safety and hygiene. These include the well-being of workers, products, and the industry. It not only helps to reduce risks, but also helps to lower expenses.
Conclusion
Thus, we have seen that there are a variety of regulations for chemicals in the European Union. REACH and CLP regulations are the most important ones in force in EU. In addition to that, there are some more regulations like BPR, PIC, POPs, CAD, and CMD. In fact, the main aim of all these regulations is to ensure the safety of workers, environment, and users. Not only reduces risks, but also it provides necessary measures to keep in mind when met with an accident at the workplace. In addition, they also manage transport, storage, and recycling of chemicals. Hence, EU companies should follow these regulations.

About the Author
Rosemary Salin is an Associate Chemist at Cademix Institute of Technology, Austria with a Master of Science in Chemistry. Her interests include studying scientific problems and finding solutions to them through experiments.
Furthermore, she was a recipient of the INSPIRE (Innovation in Science Pursuit for Inspired Research) Scholarship from 2016 to 2021. It is a scholarship program by the Department of Science and Technology (DST), Government of India. It is given to deserving students doing bachelor’s and master’s courses in natural and basic sciences. Moreover, she is a member of the Cademix Career Autopilot Program. She is available for new opportunities. Please feel free to contact her.
E-mails:
rosemary.salin@cademix.org
rosemarysalin99@gmail.com
https://www.linkedin.com/in/rosemary-salin/
Keywords Related to European Union Chemical Regulations
Chemical regulations, European chemical regulations, REACH regulation, CLP regulation, Biocidal Products Regulation (BPR), PIC regulation, POPs regulation, ECHA, Chemical Agents Directive (CAD), Carcinogens and Mutagens Directive (CMD), Chemicals, Waste management, GHS, Pictograms, Safety data sheets (SDS), Chemical agents, Carcinogens, Mutagens.
REACH-IT, IUCLID, R4BP 3, SPC Editor, SAP EHS, Occupational Exposure Limit (OEL), Biological limits, Hazards, Risks, Dossier evaluation, Substance Evaluation, Substances of Very High Concern (SVHCs), Hazardous chemicals, Occupational safety and health, Flammable, Toxic substances, Labelling, Chemical products, Regulatory tools, Chemical safety, Explosive.

